The Best Screening test for Colorectal Cancer is the one that gets done!
A simple blood test for colorectal cancer screening is the preferred method for most patients of screening age.
.


37% of the U.S. screening age population are currently non-adherent to any form of colorectal cancer screening¹.

Patients who died from colorectal cancer who were not up-to-date with screening².
What is ColoHealth?
ColoHealth is an innovative blood test for colorectal cancer screening for patients unwilling or unable to be screened by other recommended methods.²
The test detects mSEPT9 DNA, a differential blood biomarker that is methylated in colorectal cancer.³
INDICATIONS FOR USE
The ColoHealth™ test is a qualitative in vitro diagnostic test for the detection of methylated Septin 9 DNA in EDTA plasma derived from patient whole blood specimens. Methylation of the target DNA sequence in the promoter region of the SEPT9 v2 transcript has been associated with the occurrence of colorectal cancer (CRC). The test uses a real-time polymerase chain reaction (PCR) with a fluorescent hydrolysis probe for the methylation-specific detection of the Septin 9 DNA target.
The ColoHealth™ test is indicated to screen adults of either sex, 50 years or older, defined as average risk for CRC, who have been offered and have a history of not completing CRC screening. Tests that are available and recommended in the USPSTF 2008 CRC screening guidelines should be offered and declined prior to offering the ColoHealth™ test. Patients with a positive ColoHealth™ test result should be referred for diagnostic colonoscopy. The ColoHealth™ test results should be used in combination with the physician’s assessment and individual risk factors in guiding patient management.
WARNINGS, LIMITATIONS, & PRECAUTIONS
The ColoHealth™ test is not intended to replace colorectal cancer screening tests that are recommended by appropriate guidelines (e.g., 2008 USPSTF guidelines) such as colonoscopy, sigmoidoscopy and high sensitivity fecal occult blood testing. The ColoHealth test is not intended for patients who are willing and able to undergo routine colorectal cancer screening tests that are recommended by appropriate guidelines.
The ColoHealth test is not intended for patients defined as having elevated risk for developing CRC based on previous history of colorectal polyps, CRC or related cancers, inflammatory bowel disease (IBD), chronic ulcerative colitis (CUC), Crohn’s disease, familial adenomatous polyposis (FAP). Peaple at higher risk also include those with a family hisotry of CRC, particularly with two or more first degree relatives with CRC, or one or more first degree relative(s) less than 50 years of age with CRC.
The ColoHealth test has not been evaluated in patients who have been diagnosed with a relevant familial (hereditary) cancer syndrome, such as non-polyposis colorectal cancer (HNPCC or Lynch Syndrome), Peutz-Jeghers Syndrome, MYH-Associated Polyposis (MAP), Gardern’s syndrome, Turcot’s (or Crail’s) syndrome, Cowden’s syndrome, Juvenile Polyposis, Cronkhite-Canada syndrome, Neurofibromatosis, or Familial Hyperplastic Polyposis, or in patients with anorectal bleeding, hematochezia, or with known iron deficiency anemia.
REFERENCES
- American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022. Atlanta, American Cancer Society; 2020.
- United States Preventive Services Task Force (USPSTF). Screening for colorectal cancer recommendations statement. Oct. 2008
- DeVos T et al. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem. 2009, 55(7):1337-1346
**The ColoHealth™ test has been developed, and the performance characteristics determined, by the New Day Diagnostics CLIA-certified laboratory performing the test. The test currently labeled as ColoHealth™ has not been cleared or approved by the US Food and Drug Administration (FDA). ColoHealth™ is a Real-Time PCR test for the detection of methylated Septin9 (mSEPT9) DNA from blood plasma specimens. It is derived from and is substantially equivalent* to the FDA-approved Epi proColon test formerly marketed by Epigenomics AG, and is the first and only FDA-approved blood test for CRC screening. ISO 13485:2016 certified, and CLIA certified. © 2024 New Day Diagnostics, LLC. All Rights Reserved
*Data on file
Why ColoHealth?
How Do Your Patients Benefit?
- For your patients who have been offered and have not completed screening by other methods, ColoHealth is a proprietary test that may result in better compliance.¹
- Simple, routine blood test.
- No stool handling
- No pretest dietary or medication restrictions before the blood draw.
- Giving the patient colorectal cancer screening options has been shown to improve patient compliance.¹

99.5% of patients who had twice declined recommended colorectal cancer screening methods, completed the ColoHealth blood test.²

Using ColoHealth
- Your patient’s blood sample may be drawn in your office or at the New Day Diagnostics clinical laboratory.
- ColoHealth has been validated for use ONLY with the BD Vacutainer K2EDTA blood collection tube.
- ColoHealth is not intended to replace colorectal cancer screening by colonoscopy, sigmoidoscopy or high-sensitivity fecal tests.
Impact of CRC Screening Non-Adherence
A blood test provides the best opportunity for an unscreened patient to participate in a screening program.
The best screening test is the one that your patient will complete.
*Higher rates have been shown in organized, navigated screening programs.
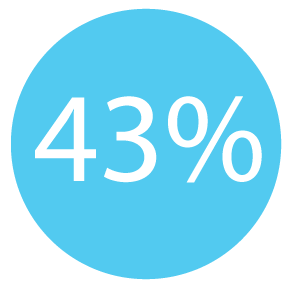
43% of new colorectal cancer cases are discovered (or found) through routine screening. ³

Patients who died from colorectal cancer who were not up-to-date with recommended screening.²
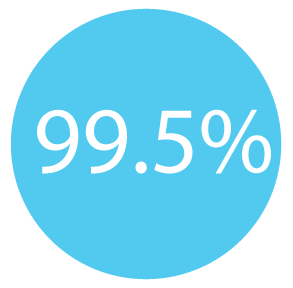
99.5% of patients who had twice declined recommended colorectal cancer screening methods, completed the ColoHealth blood test. ³¹
References
- D’Andrea, E., Ahnen, D. J., Sussman, D. A., & Najafzadeh, M. (2020). Quantifying the impact of adherence to screening strategies on colorectal cancer incidence and mortality. Cancer Medicine, 9(2), 824-836. https://doi.org/10.1002/cam4.2735.
- American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022. Atlanta, American Cancer Society; 2020.
- Doubeni C et al. (2018) Modifiable failures in the colorectal cancer screening process and their association with risk of death. Gastro. DOI: 10.1053/j.gastro.2018.09.040.
- Liles et al. (2017) Uptake of colorectal cancer screening blood test is higher than of a fecal test offered in clinic: A randomized trial. Cancer Treatment and Research Communication 10:27-31.
- Wolf, R. L., Basch, C. E., Brouse, C. H., Shmukler, C., & Shea, S. (2006). Patient preferences and adherence to colorectal cancer screening in an urban population. American journal of public health, 96(5), 809-811.
HOW DO MY PATIENTS GET TESTED?
Provider-Patient Discussion
Routine Blood Draw
Plasma-based Testing by Real-Time PCR

Patient Management
get screened with colohealth in three easy steps
ANSWER PATIENT QUESTIONS
ORDER COLOHEALTH ONLINE
SCHEDULE BLOOD DRAW
Understanding ColoHealth Test Results
NEGATIVE TEST RESULT
If ColoHealth is negative, discuss a future screening plan that is right for your patient.
POSITIVE TEST RESULT
If ColoHealth is positive, refer for diagnostic colonoscopy.
TECHNICAL INFORMATION FOR HEALTHCARE PROFESSIONALS
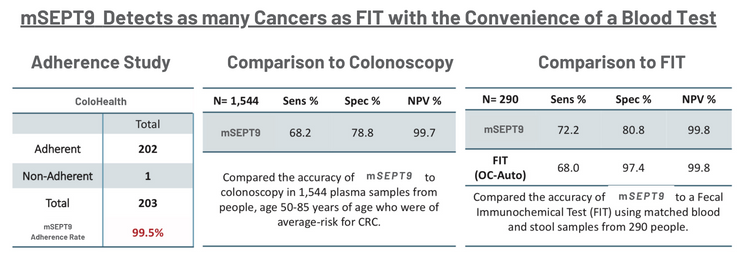
ColoHealth detects as many cancers as FIT with the convenience of a blood test.¹,²
What is the science behind ColoHealth?
- ColoHealth detects methylated Septin 9 DNA that has been shed into the bloodstream from proximal and distal colon and rectal sites.
- Methylation of the SEPT9 gene is increased in colorectal cancer.

Where do my patients get tested?
A routine blood draw may be performed at your office or New Day Diagnostics’ clinical laboratory.
Important Coding Information
CPT Code: 81327
DEX Z Code (TM) Identifier: ZB22FC
-
References
- Johnson D et al. (2014) Plasma Septin 9 versus fecal immunochemical testing for colorectal cancer screening: a prospective multicenter study. PLOS ONE 9(6) e98238.
- Potter N et al. (2014) Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin Chem 60(9):1183-1191.
*Data generated with Epi Pro Colon



